Stoichiometry mole to mole worksheet answers provide a structured approach to understanding the fundamental principles of stoichiometry, empowering learners to navigate chemical reactions with precision and confidence. This comprehensive guide delves into the intricacies of mole-to-mole conversions, chemical equation balancing, and stoichiometry calculations, equipping readers with the knowledge and skills to excel in chemistry.
The significance of stoichiometry extends beyond the classroom, with applications in diverse fields such as medicine, engineering, and environmental science. By mastering the concepts presented in this worksheet, learners gain a solid foundation for success in these and other disciplines.
Stoichiometry Mole-to-Mole Worksheet Answers: Stoichiometry Mole To Mole Worksheet Answers
Introduction, Stoichiometry mole to mole worksheet answers
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It is essential for understanding the behavior of chemical systems and for making predictions about the outcome of reactions.
A mole-to-mole worksheet is a tool that can be used to practice stoichiometry calculations. These worksheets typically contain a series of problems that require students to convert between moles and number of particles, balance chemical equations, and calculate the amount of reactants and products in a chemical reaction.
Mole-to-Mole Conversions
A mole is a unit of measurement that is used to express the amount of a substance. One mole of a substance is equal to 6.022 x 10 23atoms, molecules, or ions of that substance.
To convert between moles and number of particles, you can use the following formulas:
- Number of particles = moles x 6.022 x 10 23
- Moles = number of particles / 6.022 x 10 23
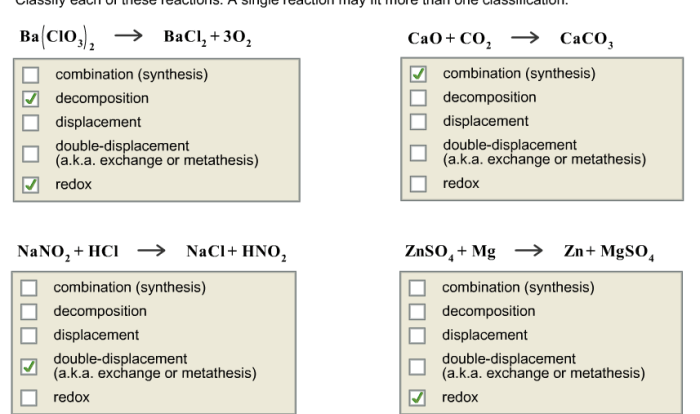
Balancing Chemical Equations
A chemical equation is a symbolic representation of a chemical reaction. In order for a chemical equation to be balanced, the number of atoms of each element must be the same on both sides of the equation.
To balance a chemical equation, you can use the following steps:
- Write the unbalanced equation.
- Count the number of atoms of each element on both sides of the equation.
- Add coefficients to the reactants and products to balance the number of atoms of each element.
Stoichiometry Calculations
Stoichiometry calculations can be used to determine the amount of reactants and products that are involved in a chemical reaction.
To perform a stoichiometry calculation, you can use the following steps:
- Balance the chemical equation.
- Convert the given amount of one reactant to moles.
- Use the mole-to-mole ratio from the balanced equation to convert the moles of the reactant to moles of the product.
- Convert the moles of the product to the desired units.
Mole-to-Mole Worksheet Practice
| Problem | Solution |
|---|---|
| Convert 2.5 moles of NaCl to number of particles. | 2.5 moles x 6.022 x 1023 particles/mole = 1.5055 x 1024 particles |
| Balance the following equation: Fe + HCl → FeCl2 + H2 | 2Fe + 6HCl → 2FeCl2 + 3H2 |
| Calculate the mass of MgO that is produced when 5.0 g of Mg reacts with excess O2. | 5.0 g Mg x (1 mole Mg / 24.31 g Mg) x (1 mole MgO / 1 mole Mg) x (40.30 g MgO / 1 mole MgO) = 8.38 g MgO |
Applications of Stoichiometry
Stoichiometry is used in a wide variety of fields, including medicine, engineering, and environmental science.
In medicine, stoichiometry is used to calculate the dosage of drugs and to determine the amount of nutrients that are needed by the body.
In engineering, stoichiometry is used to design chemical processes and to predict the performance of chemical reactors.
In environmental science, stoichiometry is used to study the chemical reactions that occur in the environment and to develop strategies for pollution control.
Commonly Asked Questions
What is the purpose of a mole-to-mole worksheet?
A mole-to-mole worksheet provides a structured framework for practicing mole-to-mole conversions, chemical equation balancing, and stoichiometry calculations, reinforcing understanding of these fundamental concepts.
How can I use mole-to-mole ratios to balance chemical equations?
Mole-to-mole ratios indicate the stoichiometric proportions of reactants and products in a chemical reaction. By comparing these ratios, you can adjust the coefficients in the equation to ensure that the number of atoms of each element is balanced on both sides.
What are some real-world applications of stoichiometry?
Stoichiometry plays a crucial role in fields such as medicine (determining drug dosages), engineering (designing chemical processes), and environmental science (assessing pollution levels). Understanding stoichiometry is essential for addressing real-world challenges and making informed decisions.