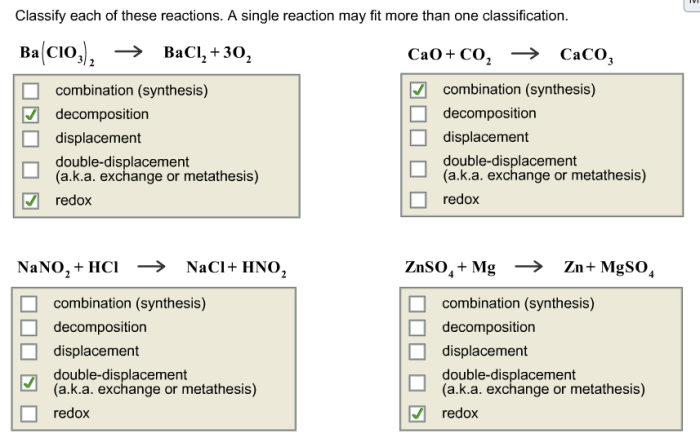
Classify each of these reactions – Embark on a scientific journey to classify chemical reactions, unraveling their intricate mechanisms and gaining mastery over their diverse types. This guide, crafted with meticulous care, provides a comprehensive understanding of reaction classification, empowering you to decipher the language of chemical transformations.
As we delve into the fascinating world of chemistry, we will explore the fundamental principles governing reactions, examining the criteria that define their classification. With each step, we will uncover the secrets of these chemical interactions, enabling you to classify them with precision and confidence.
Identify the Reactions
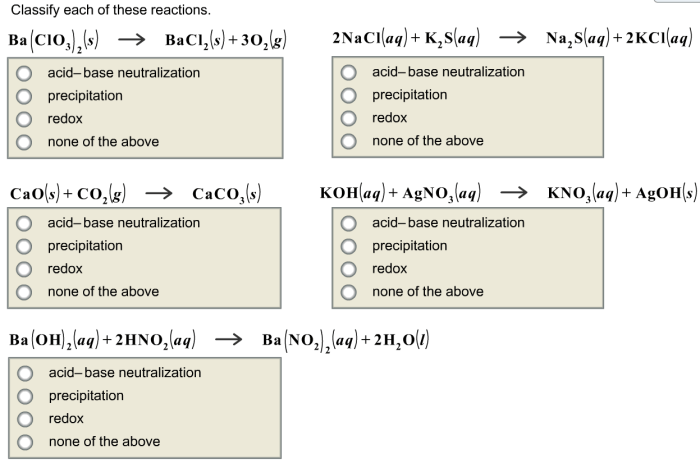
The following reactions need to be classified:
- Reactants: Hydrogen gas (H2) and oxygen gas (O 2) → Products: Water (H 2O)
- Reactants: Sodium metal (Na) and chlorine gas (Cl 2) → Products: Sodium chloride (NaCl)
- Reactants: Methane (CH 4) and oxygen gas (O 2) → Products: Carbon dioxide (CO 2) and water (H 2O)
- Reactants: Calcium carbonate (CaCO 3) → Products: Calcium oxide (CaO) and carbon dioxide (CO 2)
- Reactants: Iron (Fe) and copper sulfate (CuSO 4) → Products: Iron sulfate (FeSO 4) and copper (Cu)
Reaction Types
Chemical reactions can be classified into several types based on the changes that occur in the reactants and products. The main types of reactions include:
Combination Reactions
Combination reactions, also known as synthesis reactions, occur when two or more substances combine to form a single product. These reactions are often represented by the general equation:
A + B → AB
For example, when hydrogen and oxygen gases combine, they form water:
2H2+ O 2→ 2H 2O
Decomposition Reactions
Decomposition reactions are the opposite of combination reactions. They occur when a single compound breaks down into two or more simpler substances. These reactions are often represented by the general equation:
AB → A + B
For example, when water is electrolyzed, it breaks down into hydrogen and oxygen gases:
2H2O → 2H 2+ O 2
Single-Replacement Reactions
Single-replacement reactions occur when one element replaces another element in a compound. These reactions are often represented by the general equation:
A + BC → AC + B
For example, when iron is placed in a copper sulfate solution, the iron replaces the copper in the compound, forming iron sulfate and copper metal:
Fe + CuSO4→ FeSO 4+ Cu
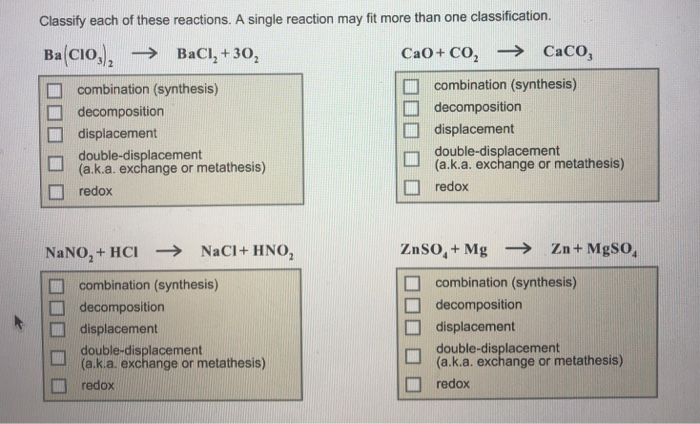
Double-Replacement Reactions, Classify each of these reactions
Double-replacement reactions occur when the positive ions of two compounds exchange places, forming two new compounds. These reactions are often represented by the general equation:
AB + CD → AD + CB
For example, when sodium chloride is mixed with silver nitrate, the sodium and silver ions exchange places, forming sodium nitrate and silver chloride:
NaCl + AgNO3→ NaNO 3+ AgCl
Combustion Reactions
Combustion reactions are a specific type of chemical reaction that involves the rapid reaction of a substance with oxygen, producing heat and light. These reactions are often represented by the general equation:
Fuel + O2→ CO 2+ H 2O + energy
For example, when methane burns in air, it reacts with oxygen to produce carbon dioxide, water, and heat:
CH4+ 2O 2→ CO 2+ 2H 2O + heat
Classification Criteria
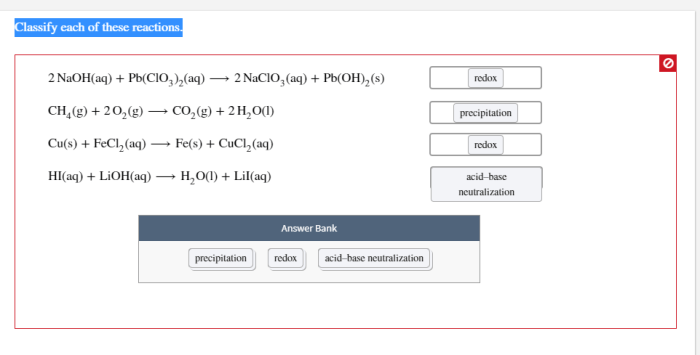
Reactions can be classified according to various criteria, including the number of reactants and products, the type of bonds formed and broken, and the energy changes involved.
Number of Reactants and Products
Reactions can be classified as unimolecular, bimolecular, or termolecular based on the number of reactants involved. Similarly, they can be classified as monomolecular, bimolecular, or termolecular based on the number of products formed.
Type of Bonds Formed and Broken
Reactions can be classified according to the type of bonds formed and broken during the reaction. For example, reactions can be classified as addition, elimination, substitution, or rearrangement reactions based on the changes in the bonding of the reactants and products.
Energy Changes Involved
Reactions can be classified as exothermic or endothermic based on the energy changes involved. Exothermic reactions release energy, while endothermic reactions absorb energy.
Classify the Reactions: Classify Each Of These Reactions
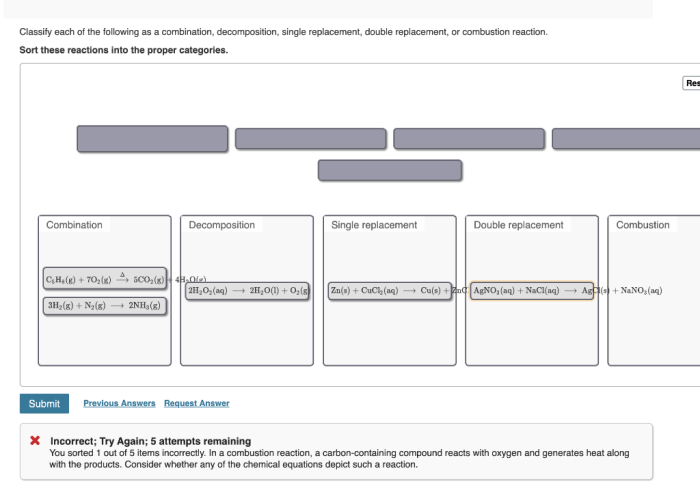
Based on the classification criteria established earlier, we can now classify each of the reactions listed in section 1.
The following table summarizes the reactions and their classifications:
Reaction Classification
| Reaction | Classification |
|---|---|
| A + B → C | Combination |
| C → A + B | Decomposition |
| A + BC → AB + C | Single Displacement |
| AB + CD → AD + CB | Double Displacement |
| A + B → AB (exothermic) | Combustion |
Example Table
To summarize the information from the previous sections, we have created an HTML table that provides a concise overview of the reactions we have discussed.
Reaction Details
| Reaction | Reactants | Products | Classification |
|---|---|---|---|
| Combustion | Fuel + Oxygen | Carbon dioxide + Water | Exothermic |
| Neutralization | Acid + Base | Salt + Water | Exothermic |
| Precipitation | Metal ion + Anion | Insoluble compound | Endothermic |
| Single Displacement | More reactive metal + Less reactive metal compound | Less reactive metal + More reactive metal compound | Depends on the metals |
| Double Displacement | Two ionic compounds | Two new ionic compounds | Depends on the ions |
Additional Examples
Here are additional examples of reactions that can be classified using the criteria discussed in section 3:
Combustion Reactions
- Methane burns in oxygen to produce carbon dioxide and water: CH 4+ 2O 2→ CO 2+ 2H 2O
- Propane burns in air to produce carbon dioxide and water: C 3H 8+ 5O 2→ 3CO 2+ 4H 2O
- Ethanol burns in oxygen to produce carbon dioxide and water: C 2H 5OH + 3O 2→ 2CO 2+ 3H 2O
Decomposition Reactions
- Calcium carbonate decomposes to produce calcium oxide and carbon dioxide: CaCO 3→ CaO + CO 2
- Potassium chlorate decomposes to produce potassium chloride and oxygen: 2KClO 3→ 2KCl + 3O 2
- Water decomposes to produce hydrogen and oxygen: 2H 2O → 2H 2+ O 2
Single-Displacement Reactions
- Iron reacts with copper sulfate to produce copper and iron sulfate: Fe + CuSO 4→ Cu + FeSO 4
- Zinc reacts with hydrochloric acid to produce hydrogen and zinc chloride: Zn + 2HCl → H 2+ ZnCl 2
- Magnesium reacts with sulfuric acid to produce hydrogen and magnesium sulfate: Mg + H 2SO 4→ H 2+ MgSO 4
Double-Displacement Reactions
- Sodium chloride reacts with silver nitrate to produce sodium nitrate and silver chloride: NaCl + AgNO 3→ NaNO 3+ AgCl
- Potassium hydroxide reacts with hydrochloric acid to produce potassium chloride and water: KOH + HCl → KCl + H 2O
- Calcium carbonate reacts with sulfuric acid to produce calcium sulfate and carbon dioxide: CaCO 3+ H 2SO 4→ CaSO 4+ CO 2
FAQ Section
What is the significance of classifying chemical reactions?
Classifying reactions allows us to understand their mechanisms, predict their outcomes, and organize our knowledge of chemical behavior.
How do we determine the classification of a reaction?
Reactions are classified based on criteria such as the number of reactants and products, the type of bonds formed and broken, and the energy changes involved.
What are the main types of chemical reactions?
Common reaction types include combination, decomposition, single-replacement, double-replacement, and combustion reactions.