The molecular geometry POGIL answer key provides a comprehensive overview of the principles and applications of molecular geometry, offering students a valuable resource for understanding the three-dimensional arrangement of atoms in molecules.
This guide explores the fundamental concepts of molecular geometry, including VSEPR theory, molecular orbitals, hybridization, and polarity. It also delves into the applications of molecular geometry in chemistry, showcasing its importance in predicting the properties and behavior of molecules.
Introduction to Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number and type of atoms in the molecule, as well as the hybridization of the central atom.
Molecular geometry is important because it affects many properties of a molecule, such as its polarity, reactivity, and physical state. For example, linear molecules are typically nonpolar, while bent molecules are polar. Linear molecules also tend to be more reactive than bent molecules.
Examples of Molecular Geometries
- Linear: Two atoms bonded together in a straight line, with a bond angle of 180 degrees.
- Trigonal planar: Three atoms bonded to a central atom in a flat, triangular shape, with bond angles of 120 degrees.
- Tetrahedral: Four atoms bonded to a central atom in a three-dimensional, tetrahedral shape, with bond angles of 109.5 degrees.
- Bent: Three atoms bonded to a central atom in a V-shape, with bond angles less than 120 degrees.
- T-shaped: Five atoms bonded to a central atom in a T-shape, with bond angles of 90 degrees and 180 degrees.
- Square planar: Four atoms bonded to a central atom in a square, flat shape, with bond angles of 90 degrees.
- Octahedral: Six atoms bonded to a central atom in an octahedral shape, with bond angles of 90 degrees.
VSEPR Theory
Valence Shell Electron Pair Repulsion (VSEPR) theory predicts the geometry of molecules based on the repulsion between electron pairs in the valence shell of the central atom.
Predicting Molecular Geometry
To predict the molecular geometry using VSEPR theory, follow these steps:
- Determine the number of valence electrons in the molecule.
- Arrange the valence electrons into electron pairs.
- Determine the number of bonding and non-bonding electron pairs.
- Apply the following rules to predict the molecular geometry:
- Lone pairs repel each other more strongly than bonding pairs.
- Electron pairs arrange themselves to minimize repulsion.
- The molecular geometry is determined by the positions of the bonding pairs only.
Molecular Orbitals
Molecular orbitals (MOs) are mathematical functions that describe the wave-like behavior of electrons in a molecule. They are formed by the combination of atomic orbitals, which are the orbitals of individual atoms.
The shape and energy of MOs are determined by the number and type of atomic orbitals that combine to form them. The lowest energy MOs are formed by the combination of atomic orbitals with similar energies and shapes. These MOs are bonding orbitals, which means that they lead to the formation of chemical bonds between atoms.
Molecular Geometry
The shape of a molecule is determined by the arrangement of its MOs. The MOs with the lowest energy are filled first, and these electrons are responsible for forming the chemical bonds between atoms. The shape of the molecule is then determined by the repulsion between the electrons in the filled MOs.
For example, in a molecule of methane (CH 4), the four hydrogen atoms are arranged in a tetrahedral shape around the carbon atom. This shape is due to the repulsion between the electrons in the filled MOs of the carbon atom.
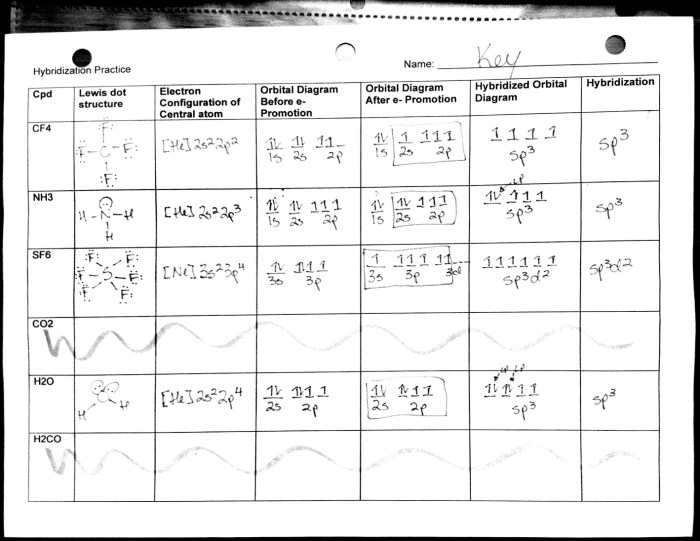
Hybridization
Hybridization is the process of combining atomic orbitals to form new hybrid orbitals with different shapes and energies. These hybrid orbitals are used to form chemical bonds and determine the molecular geometry of a molecule.
The type of hybridization that occurs depends on the number and type of atomic orbitals that are involved. For example, in carbon, the 2s and three 2p orbitals hybridize to form four equivalent sp 3hybrid orbitals. These sp 3hybrid orbitals have a tetrahedral shape and are used to form four single bonds with other atoms.
Effects of Hybridization on Molecular Geometry
Hybridization has a significant impact on molecular geometry. The shape of a molecule is determined by the arrangement of its valence electrons in the hybrid orbitals. For example, a molecule with sp 3hybrid orbitals will have a tetrahedral shape, while a molecule with sp 2hybrid orbitals will have a trigonal planar shape.
- sp3hybridization: Tetrahedral geometry (e.g., CH 4)
- sp2hybridization: Trigonal planar geometry (e.g., C 2H 4)
- sp hybridization:Linear geometry (e.g., C 2H 2)
The hybridization of atomic orbitals is a fundamental concept in chemistry that helps to explain the structure and bonding of molecules.
Polarity and Molecular Geometry
The polarity of a molecule is determined by the distribution of its electrons. A molecule is polar if it has a net positive or negative charge. The molecular geometry of a molecule is determined by the arrangement of its atoms.
The polarity of a molecule and its molecular geometry are related because the distribution of electrons in a molecule affects the way that the atoms are arranged.
Polar Molecules
A molecule is polar if it has a net positive or negative charge. This can happen if the molecule has an uneven distribution of electrons. For example, the molecule HCl is polar because the chlorine atom has a higher electronegativity than the hydrogen atom.
This means that the electrons in the HCl molecule are pulled towards the chlorine atom, giving the molecule a net negative charge at the chlorine end and a net positive charge at the hydrogen end.
Nonpolar Molecules, Molecular geometry pogil answer key
A molecule is nonpolar if it has no net positive or negative charge. This can happen if the molecule has an even distribution of electrons. For example, the molecule CH 4is nonpolar because the four hydrogen atoms are arranged symmetrically around the carbon atom.
This means that the electrons in the CH 4molecule are evenly distributed, giving the molecule no net charge.
Resonance and Molecular Geometry
Resonance is a concept in chemistry that describes the delocalization of electrons within a molecule or polyatomic ion. It occurs when two or more Lewis structures can be drawn for a molecule, and none of them can fully represent the molecule’s structure.
When resonance occurs, the molecule’s geometry is affected. The molecule’s shape is determined by the arrangement of its atoms and the number of electron pairs around each atom. Resonance can lead to a more symmetrical molecule, as the electron pairs are delocalized and spread out over a larger area.
Effects of Resonance on Molecular Geometry
The effects of resonance on molecular geometry can be significant. For example, in the case of benzene, the resonance between the two Kekule structures leads to a symmetrical hexagonal shape. This shape is more stable than the shape of a cyclohexatriene molecule, which has alternating single and double bonds.
Another example of the effects of resonance on molecular geometry is the case of the carbonate ion. The resonance between the three Lewis structures leads to a symmetrical trigonal planar shape. This shape is more stable than the shape of a molecule with a single carbon-oxygen double bond and two carbon-oxygen single bonds.
Applications of Molecular Geometry
Molecular geometry plays a crucial role in chemistry as it enables the prediction of various properties and behaviors of molecules. By understanding the spatial arrangement of atoms within a molecule, scientists can gain insights into its reactivity, polarity, and physical properties.
Predicting Molecular Properties
Molecular geometry directly influences the physical properties of molecules, such as their melting and boiling points. For instance, molecules with symmetrical geometries, such as tetrahedral or octahedral, tend to have higher melting and boiling points due to stronger intermolecular forces.
In contrast, molecules with irregular geometries have lower melting and boiling points because of weaker intermolecular forces.
Chemical Reactivity
The geometry of a molecule also affects its chemical reactivity. Molecules with specific geometries are more likely to undergo certain reactions than others. For example, molecules with trigonal planar geometry, such as carbon dioxide, are more reactive towards nucleophilic addition reactions.
This is because the nucleophile can easily approach the electrophilic carbon atom due to the absence of steric hindrance.
Biological Applications
Molecular geometry is essential in understanding biological processes. The shape and geometry of biomolecules, such as proteins and DNA, determine their function and interactions with other molecules. For instance, the double-helical structure of DNA is a result of the specific molecular geometry of its nucleotide building blocks.
Essential Questionnaire: Molecular Geometry Pogil Answer Key
What is the VSEPR theory?
The VSEPR theory (Valence Shell Electron Pair Repulsion) is a model used to predict the molecular geometry of a molecule based on the number of valence electron pairs around the central atom.
How does hybridization affect molecular geometry?
Hybridization is the mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies. It affects molecular geometry by determining the spatial arrangement of the electron pairs around the central atom.
What is the relationship between polarity and molecular geometry?
Polarity refers to the uneven distribution of electrons within a molecule. Molecular geometry influences polarity by determining the direction and magnitude of the dipole moment, which is a measure of the polarity of a molecule.